High pressure treatment
High pressure treatment for the inactivation of microorganisms have been the subject of research for some time and are already being used successfully in specific areas of industrial practice. In classic hydrostatic high-pressure processes (HHP), the materials to be treated are exposed to pressures of 6,000-10,000 bar (600 – 1000 MPa) in order to kill microorganisms [1]. The materials to be treated are usually liquids or dissolved substances. However, it is also possible to treat solids, provided they can be introduced into a liquid (e.g. water). However, the inactivating effect on microorganisms depends on the type of microorganism. Some special types of bacteria withstand HHP treatment very well [2]. In addition to HHP, there are other high-pressure processes such as the high-pressure homogenization of liquid foods [3].
The advantage of such high-pressure processes is the inactivation of microorganisms at low temperatures (ambient temperature), without the use of disinfectants/biocides and without chemically altering or changing the quality of the treated products. The original properties of the treated goods are affected only slightly by the processes [4]. These processes are therefore considered to be residue-free and particularly mild. For this reason, high-pressure processes are used in the treatment of foodstuffs, spices, pharmaceutical preparations, etc. A well-known everyday application is the high-pressure treatment of high-quality and sensitive products, such as fresh direct juices or fruit purees, whose taste would be altered too much by heating [5].
These properties make HPP and similar processes interesting in principle for the treatment of wastewater in order to specifically reduce the microbial load and also kill multi-resistant pathogens. These processes have the advantage that no disinfectants are used and therefore no additional contamination with chemical substances occurs. However, generating extremely high pressures is technically demanding and places high challenges on technology and materials. An alternative to HHP are processes that rely on compressed gases such as carbon dioxide (CO2) or nitrogen and show a comparable inactivation effect even at comparably lower pressures of less than 100 bar. In this area, so-called dense phase carbon dioxide (DPCO2 or DPCD) has proven to be particularly effective [6].
DPCO2
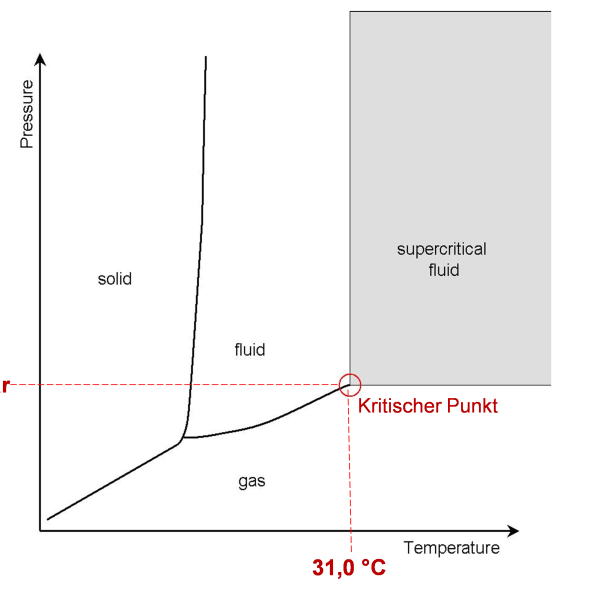
Carbon dioxide (CO2) is a gas that occurs in the Earth’s atmosphere at a concentration of approx. 426 ppm (0.0426 %, January 2025, NOAA). The gas enters the atmosphere through geological processes or mainly through the oxidation of organic carbon by technical and biological processes. CO2 is a product of many metabolic pathways, such as cellular respiration, and is therefore excreted by most living organisms. For example, the exhaled air of humans has a CO2 content of 3-6 %. The density of gaseous CO2 is higher than that of air, so CO2 sinks to the ground. It is odorless and tasteless, but is readily soluble in water and forms carbonic acid in aqueous solution, which leads to a decrease in the pH value. However, this only makes up a small proportion of the dissolved gas. CO2 sublimates from the solid to the gaseous phase at atmospheric pressure at -78.5 °C without becoming a liquid. Only at a pressure of 5.2 bar and a temperature of < -56.6 °C, at the so-called triple point, does CO2 condense into a liquid. This is referred to in the application as “liquid CO2”, LCO2. The critical point of CO2 is reached at 73.8 bar and 31.0 °C, where it enters the supercritical state (“supercritical CO2”, SCCO2). In this state, the phase boundaries disappear and SCCO2 exhibits the properties of both a gas and a liquid: it has the low viscosity of gaseous CO2 and can therefore penetrate the finest pores of solid materials very easily, but at the same time it is a good solvent for non-polar organic substances. A schematic phase diagram is shown in Fig. 1. The term “compressed carbon dioxide” (dense phase carbon dioxide, DPCO2) generally includes liquid carbon dioxide (LCO2) or supercritical carbon dioxide (SCCO2).
It has been observed for a long time that CO2 has antimicrobial properties. Although the gas is not toxic, CO2 concentrations of >8% can lead to suffocation within 30-60 minutes. The “inhibitory effects on microbes” were first summarized in 1927 [7]. The antimicrobial effect caused by the displacement of oxygen is an effect that has been used for thousands of years. It plays a role during the fermentation of mashes in wine and beer making, as the CO2 produced during fermentation prevents aerobic microorganisms such as molds, which can cause spoilage, from growing before the alcohol content is high enough. In addition, the formation of carbon dioxide inhibits the growth of microorganisms. The addition of carbon dioxide to beverages not only improves the taste, but also extends the shelf life. This effect has been used in the beverage industry for a long time [8].
However, compressed CO2 also has antimicrobial properties in direct technical applications. The first studies describing inactivation by compressed CO2 were published as early as 1951 [9]. Interest in DPCO2 processes has increased significantly since the 1990s. Numerous publications report efficacy against bacteria [2], [10], [11], [12], [13], [14], [15], [16], [17], [18], fungi [19], [20], [21], [22], [23], occasionally bacterial endospores [11], [24], [25] and viruses [26], [27]. A reduction factor up to 9 (1:billion) was observed for inactivation depending on microorganisms used, phase (LCO2/SCCO2), temperature and pressure, and incubation time.
The exact mechanism by which DPCO2 sterilizes microorganisms is not yet fully understood. Various investigations have revealed several effects underlying the antimicrobial effect of compressed carbon dioxide. The following effects have been identified in various studies:
• Acidification of the surrounding environment: If water is present during the incubation of microorganisms in DPCO2, carbonic acid (H2CO3) is formed. This acidifies the surrounding environment. Acidification disrupts the proton gradients that are important for the microorganisms and thus significantly impairs the metabolism of the cells [28].
• The diffusion of the compressed CO2 into the cell membrane, which consists of phospholipids, leads to a decrease in membrane fluidity. This significantly disrupts the structure and function of the membrane, making it more permeable to other substances. As a result, cytotoxic substances can penetrate the cell more easily and vital substances can diffuse out of the cell. The cells can no longer maintain their natural state of metabolic equilibrium [29], [30], [31].
• DPCO2 is an excellent solvent. This allows intracellular cell components to be dissolved and extracted from the cell. The leaching of certain substances into the surrounding medium could be detected [32].
• Compressed CO2 penetrates through the cell membrane into the cytoplasm of microorganisms and leads to an intracellular increase of the acidity after the formation of carbonic acid. However, the pH decline cannot be compensated for in the case of massive CO2 influx and then leads to a reduction to pH=3.3. The reduction of the intracellular pH value leads, among other things, to the inactivation of pH-sensitive key enzymes of cell metabolism [33], [34].
• The formation of carbonic acid in the cells also leads to a change in the ion concentration. Bivalent metal ions such as Mg2+ and Ca2+ can be deposited through the formation of poorly soluble carbonate salts and their precipitation. These are then missing in the metabolism as gradients or as cofactors in enzymes. As a result, the processes in the cell are severely disrupted [35].
• It is also being debated whether the decompression of DPCO2 causes cell ruptures, as the CO2 diffused into the cell under high pressure expands during sudden depressurization and passes into the gas phase [31]. Damaged membranes can be seen in electron microscope examinations. Whether this effect is really relevant is the subject of debate, as very little protein material escapes from the treated cells [36].
All of these effects occur simultaneously and can be expected to work synergistically.
The use of DPCO2 in technological applications is advantageous for many reasons: it is non-flammable, non-explosive, non-toxic, non-allergenic, evaporates without residue, inexpensive, readily available and recyclable. Disinfection with DPCO2 is considered a non-thermal process and is also known as cold pasteurization. Because it takes place at low temperatures, it can also be considered energy efficient.
While DPCO2 disinfection is being investigated and developed for many other applications, DPCO2 is already used in several industrial applications, such as extraction of natural products (e.g., lupulin from hops, caffeine from coffee), impregnation of wood, coloring of plastics, and removal of pesticides from rice. As a result, a wide range of technological/apparatus knowledge is available for large-scale industrial implementation.
All of these properties make the use of DPCO2 for wastewater treatment interesting, in order to reduce the number of multi-resistant pathogens in a sustainable way.
# Selected literature references:
[1] S. Koseki, M. Matsubara, und K. Yamamoto, „Prediction of a Required Log Reduction with Probability for Enterobacter sakazakii during High-Pressure Processing, Using a Survival/Death Interface Model“, Appl. Environ. Microbiol., Bd. 75, Nr. 7, S. 1885–1891, Apr. 2009, doi: 10.1128/AEM.02283-08.
[2] H. Gollwitzer u. a., „High Hydrostatic Pressure for Disinfection of Bone Grafts and Biomaterials: An Experimental Study“, Open Orthop. J., Bd. 3, Nr. 1, S. 1–7, Jan. 2009, doi: 10.2174/1874325000903010001.
[3] R. Levy, Z. Okun, und A. Shpigelman, „High-Pressure Homogenization: Principles and Applications Beyond Microbial Inactivation“, Food Eng. Rev., Bd. 13, Nr. 3, S. 490–508, Sep. 2021, doi: 10.1007/s12393-020-09239-8.
[4] R. Sehrawat, B. P. Kaur, P. K. Nema, S. Tewari, und L. Kumar, „Microbial inactivation by high pressure processing: principle, mechanism and factors responsible“, Food Sci. Biotechnol., Bd. 30, Nr. 1, S. 19–35, Jan. 2021, doi: 10.1007/s10068-020-00831-6.
[5] V. Chiozzi, S. Agriopoulou, und T. Varzakas, „Advances, Applications, and Comparison of Thermal (Pasteurization, Sterilization, and Aseptic Packaging) against Non-Thermal (Ultrasounds, UV Radiation, Ozonation, High Hydrostatic Pressure) Technologies in Food Processing“, Appl. Sci., Bd. 12, Nr. 4, S. 2202, Feb. 2022, doi: 10.3390/app12042202.
[6] H. T. Vo, T. Imai, T. T. Ho, T.-L. T. Dang, und S. A. Hoang, „Potential application of high pressure carbon dioxide in treated wastewater and water disinfection: Recent overview and further trends“, J. Environ. Sci., Bd. 36, S. 38–47, Okt. 2015, doi: 10.1016/j.jes.2015.04.006.
[7] G. Valley und L. F. Rettger, „THE INFLUENCE OF CARBON DIOXIDE ON BACTERIA“, J. Bacteriol., Bd. 14, Nr. 2, S. 101–137, Aug. 1927, doi: 10.1128/jb.14.2.101-137.1927.
[8] J. R. Donald, C. L. Jones, und A. R. M. MacLean, „THE EFFECT OF CARBONATION ON BACTERIA IN BEVERAGES“, Am. J. Public Health, Bd. 14, Nr. 2, S. 122–128, Feb. 1924, doi: 10.2105/AJPH.14.2.122.
[9] D. Fraser, „Bursting Bacteria by Release of Gas Pressure“, Nature, Bd. 167, Nr. 4236, S. 33–33, Jan. 1951, doi: 10.1038/167033a0.
[10] C. I. Wei, M. O. Balaban, S. Y. Fernando, und A. J. Peplow, „Bacterial Effect of High Pressure CO2 Treatment on Foods Spiked with Listeria or Salmonella“, J. Food Prot., Bd. 54, Nr. 3, S. 189–193, März 1991, doi: 10.4315/0362-028X-54.3.189.
[11] A. Enomoto, K. Nakamura, M. Hakoda, und N. Amaya, „Lethal effect of high-pressure carbon dioxide on a bacterial spore“, J. Ferment. Bioeng., Bd. 83, Nr. 3, S. 305–307, Jan. 1997, doi: 10.1016/S0922-338X(97)80999-3.
[12] H. Lin, N. Cao, und L. Chen, „Antimicrobial Effect of Pressurized Carbon Dioxide on Listeria monocytogenes“, J. Food Sci., Bd. 59, Nr. 3, S. 657–659, Mai 1994, doi: 10.1111/j.1365-2621.1994.tb05587.x.
[13] A. K. Dillow, F. Dehghani, J. S. Hrkach, N. R. Foster, und R. Langer, „Bacterial inactivation by using near- and supercritical carbon dioxide“, Proc. Natl. Acad. Sci., Bd. 96, Nr. 18, S. 10344–10348, Aug. 1999, doi: 10.1073/pnas.96.18.10344.
[14] F. Dehghani, N. Annabi, M. Titus, P. Valtchev, und A. Tumilar, „Sterilization of ginseng using a high pressure CO2 at moderate temperatures“, Biotechnol. Bioeng., Bd. 102, Nr. 2, S. 569–576, Feb. 2009, doi: 10.1002/bit.22059.
[15] C. Cinquemani, C. Boyle, E. Bach, und E. Schollmeyer, „Inactivation of microbes using compressed carbon dioxide—An environmentally sound disinfection process for medical fabrics“, J. Supercrit. Fluids, Bd. 42, Nr. 3, S. 392–397, Okt. 2007, doi: 10.1016/j.supflu.2006.11.001.
[16] L. Garcia-Gonzalez u. a., „Membrane permeabilization and cellular death of Escherichia coli, Listeria monocytogenes and Saccharomyces cerevisiae as induced by high pressure carbon dioxide treatment“, Food Microbiol., Bd. 27, Nr. 4, S. 541–549, Juni 2010, doi: 10.1016/j.fm.2009.12.004.
[17] A. Arbal u. a., „Dense phase carbon dioxide (DPCD) inactivation of microorganisms and enzymes, and its application in food: A review“, Food Chem. Adv., Bd. 5, S. 100782, Dez. 2024, doi: 10.1016/j.focha.2024.100782.
[18] T. Hochmuth, „Entwicklung neuartiger bioresorbierbarer Implantatmaterialien sowie kompatibler Sterilisationsverfahren“, wfk – cleaning technologies e.V., Krefeld; Dresden, Schlussbericht IGF-17455 BG, Sep. 2014.
[19] G. Ferrentino und S. Spilimbergo, „Non-thermal pasteurization of apples in syrup with dense phase carbon dioxide“, J. Food Eng., Bd. 207, S. 18–23, Aug. 2017, doi: 10.1016/j.jfoodeng.2017.03.014.
[20] G. J. Haas, H. E. Prescott, E. Dudley, R. Dik, C. Hintlian, und L. Keane, „INACTIVATION OF MICROORGANISMS BY CARBON DIOXIDE UNDER PRESSURE“, J. Food Saf., Bd. 9, Nr. 4, S. 253–265, Jan. 1989, doi: 10.1111/j.1745-4565.1989.tb00525.x.
[21] A. Bernhardt u. a., „Improved Sterilization of Sensitive Biomaterials with Supercritical Carbon Dioxide at Low Temperature“, PLOS ONE, Bd. 10, Nr. 6, S. e0129205, Juni 2015, doi: 10.1371/journal.pone.0129205.
[22] D. Mantoan und S. Spilimbergo, „Mathematical Modeling of Yeast Inactivation of Freshly Squeezed Apple Juice under High-Pressure Carbon Dioxide“, Crit. Rev. Food Sci. Nutr., Bd. 51, Nr. 1, S. 91–97, Dez. 2010, doi: 10.1080/10408390903044818.
[23] T. Parton, N. Elvassore, A. Bertucco, und G. Bertoloni, „High pressure CO2 inactivation of food: A multi-batch reactor system for inactivation kinetic determination“, J. Supercrit. Fluids, Bd. 40, Nr. 3, S. 490–496, Apr. 2007, doi: 10.1016/j.supflu.2006.07.022.
[24] J. Zhang u. a., „Sterilizing Bacillus pumilus spores using supercritical carbon dioxide“, J. Microbiol. Methods, Bd. 66, Nr. 3, S. 479–485, Sep. 2006, doi: 10.1016/j.mimet.2006.01.012.
[25] T. Watanabe u. a., „High Pressure Carbon Dioxide Decreases the Heat Tolerance of the Bacterial Spores“, Food Sci. Technol. Res., Bd. 9, Nr. 4, S. 342–344, 2003, doi: 10.3136/fstr.9.342.
[26] A. N. Efaq, N. N. N. Ab. Rahman, H. Nagao, A. A. Al-Gheethi, M. Shahadat, und M. O. Ab. Kadir, „Supercritical Carbon Dioxide as Non-Thermal Alternative Technology for Safe Handling of Clinical Wastes“, Environ. Process., Bd. 2, Nr. 4, S. 797–822, Dez. 2015, doi: 10.1007/s40710-015-0116-0.
[27] Z. Lian, D. Yang, Y. Wang, L. Zhao, L. Rao, und X. Liao, „Investigating the microbial inactivation effect of low temperature high pressure carbon dioxide and its application in frozen prawn (Penaeus vannamei)“, Food Control, Bd. 145, S. 109401, März 2023, doi: 10.1016/j.foodcont.2022.109401.
[28] S. ‐I. Hong und Y. ‐R. Pyun, „Inactivation Kinetics of Lactobacillus plantarum by High Pressure Carbon Dioxide“, J. Food Sci., Bd. 64, Nr. 4, S. 728–733, Juli 1999, doi: 10.1111/j.1365-2621.1999.tb15120.x.
[29] S. R. Kim, M. S. Rhee, B. C. Kim, H. Lee, und K. H. Kim, „Modeling of the inactivation of Salmonella typhimurium by supercritical carbon dioxide in physiological saline and phosphate-buffered saline“, J. Microbiol. Methods, Bd. 70, Nr. 1, S. 132–141, Juli 2007, doi: 10.1016/j.mimet.2007.04.003.
[30] J. Li, A. Wang, F. Zhu, R. Xu, und X. S. Hu, „Membrane Damage Induced by Supercritical Carbon Dioxide in Rhodotorula mucilaginosa“, Indian J. Microbiol., Bd. 53, Nr. 3, S. 352–358, Sep. 2013, doi: 10.1007/s12088-013-0373-4.
[31] H. T. Vo u. a., „Comparison of disinfection effect of pressurized gases of CO2, N2O, and N2 on Escherichia coli“, Water Res., Bd. 47, Nr. 13, S. 4286–4293, Sep. 2013, doi: 10.1016/j.watres.2013.04.053.
[32] S. R. Kim, H. J. Park, D. S. Yim, H. T. Kim, I.-G. Choi, und K. H. Kim, „Analysis of survival rates and cellular fatty acid profiles of Listeria monocytogenes treated with supercritical carbon dioxide under the influence of cosolvents“, J. Microbiol. Methods, Bd. 75, Nr. 1, S. 47–54, Sep. 2008, doi: 10.1016/j.mimet.2008.04.012.
[33] L. Garcia-Gonzalez u. a., „High pressure carbon dioxide inactivation of microorganisms in foods: The past, the present and the future“, Int. J. Food Microbiol., Bd. 117, Nr. 1, S. 1–28, Juni 2007, doi: 10.1016/j.ijfoodmicro.2007.02.018.
[34] S. Spilimbergo, A. Bertucco, G. Basso, und G. Bertoloni, „Determination of extracellular and intracellular pH ofBacillus subtilis suspension under CO2 treatment“, Biotechnol. Bioeng., Bd. 92, Nr. 4, S. 447–451, Nov. 2005, doi: 10.1002/bit.20606.
[35] S.-I. Hong und Y.-R. Pyun, „Membrane damage and enzyme inactivation of Lactobacillus plantarum by high pressure CO2 treatment“, Int. J. Food Microbiol., Bd. 63, Nr. 1–2, S. 19–28, Jan. 2001, doi: 10.1016/S0168-1605(00)00393-7.
[36] A. Enomoto, K. Nakamura, K. Nagai, T. Hashimoto, und M. Hakoda, „Inactivation of Food Microorganisms by High-pressure Carbon Dioxide Treatment with or without Explosive Decompression“, Biosci. Biotechnol. Biochem., Bd. 61, Nr. 7, S. 1133–1137, Jan. 1997, doi: 10.1271/bbb.61.1133.
