Bi-super catalysts
Photocatalysts enable a chemical reaction by excitation with light energy without being consumed themselves. Piezoelectric catalysts are excited by mechanical stress such as pressure or tension. In both cases, the excitation of the catalyst results in the separation of charge carriers on the catalyst’s surface, which can lead to the formation of reactive oxygen species such as hydroxyl radicals OH• and superoxide anions O2•- in an aqueous environment. These radicals can form other reactive oxygen species such as hydroperoxyl radicals HOO• and hydrogen peroxide H2O2 [1]. Hydrogen peroxide can give two hydroxyl radicals by the Fenton reaction. The radicals formed typically have a short lifetime and react directly with substrates in the immediate environment. They have an inactivating effect on microorganisms and can oxidize and thus degrade organic compounds [2]. With an oxidation potential of 2.8 V (with respect to the Standard Hydrogen Electrode (SHE)), the hydroxyl radical OH• is the most reactive oxygen species and therefore particularly interesting for advanced oxidation processes [3].
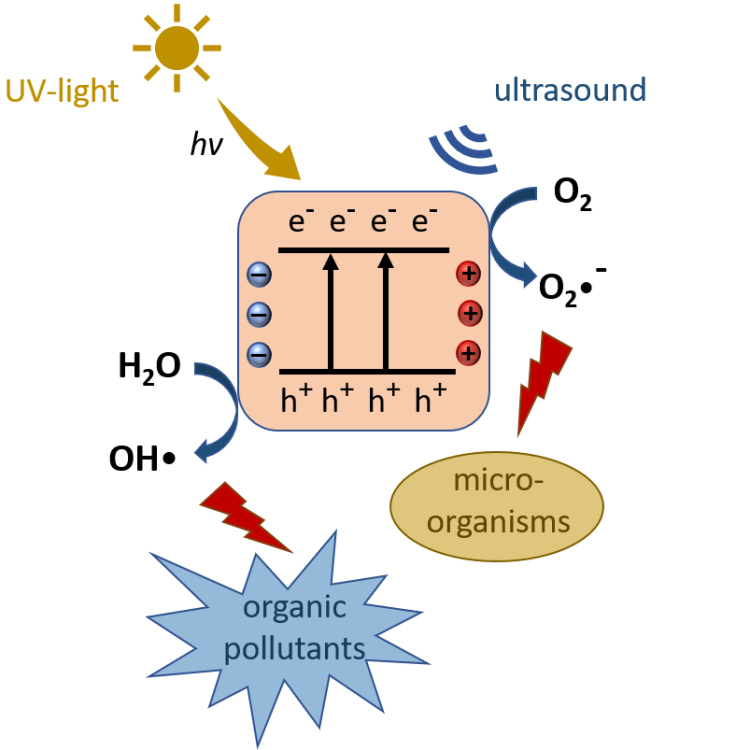
Photocatalysts such as titanium dioxide (TiO2) can directly generate hydroxyl radicals. Under the influence of ultraviolet (UV) radiation, electrons from the valence band of the photocatalyst are excited to the conduction band, leaving back electron holes in the valence band. These electron holes can react with surrounding water molecules to yield hydroxyl radicals by oxidation [4]. However, for this, the redox potential of the valence band has to be more positive than that of water (2.32 V vs. SHE) [5]. This holds true for e.g. titanium dioxide. The electrons in the conduction band can react with oxygen to produce hyperperoxide anions by reduction. To do this, the redox potential of the conduction band has to be more negative than that of oxygen (-0.16 V vs. SHE) [5]. This applies also for titanium dioxide. Electrons in the conduction band can, however, quickly recombine with the electron holes in the valence band without a chemical reaction. More than 90 % of the electron-hole pairs recombine within 10 ns after excitation and are no longer available for chemical reactions [6]. To transfer electrons from the valence band to the conduction band, the band gap must be overcome by excitation with light of an appropriate wavelength. Titanium dioxide with a band gap of 3.0-3.2 eV can be excited by UV-A radiation [7]. Photocatalysts with a lower band gap can be excited by visible light. Titanium dioxide is a prominent photocatalyst that has found numerous applications due to its advantageous properties [8].
Piezocatalysts have a non-centrosymmetric crystal structure, such as perovskites [9]. Mechanical deformation leads to the separation of charge carriers and the formation of a piezoelectric field. This can change the band gap structure and surface charge distribution of a piezocatalyst [10]. Lead zirconate titanate (PZT), barium titanate (BaTiO₃), and zinc oxide (ZnO) belong to the most studied piezocatalysts [10,11].
Bi-super catalysts combine the properties of piezoelectric catalysts and photocatalysts and can be excited both, mechanically, e.g. by ultrasonic pulses, and by UV radiation. By combining piezoelectric materials with photocatalytic systems, the efficiency and stability of photocatalytic reactions can be improved. For example, the recombination rate of charge carriers can be reduced, because the internal piezoelectric field in excited piezoelectric materials stabilizes the electrons and electron holes.
The project aims to develop novel photo-piezocatalytically active bi-super catalyst materials that enable efficient and continuous generation of reactive oxygen species. The catalysts are excited by light (e.g. UV-A), which can be produced with better efficiency and more cost-effective excitation sources than UV-C light. In addition, UV-A light is less absorbed by the aqueous media to be treated, allowing for more efficient treatment. The excitability of the bi-super catalysts by ultrasonic or high pressure pulses also allows the treatment of organically highly contaminated water qualities that are difficult to access by actinic treatment due to turbidity or high extinction (light attenuation).
[1] Kumar, P.; Vaish, R.; Sung, T. H.; Hwang, W.; Park, H. K. B.; Kumar, A.; Kebaili, I.; Boukhris, I. Effect of Poling on Photocatalysis, Piezocatalysis, and Photo–Piezo Catalysis Performance of BaBi4 Ti4 O15 Ceramics. Glob. Chall. 2023, 7 (2), 2200142. https://doi.org/10.1002/gch2.202200142.
[2] Sharma, A.; Bhardwaj, U.; Jain, D.; Kushwaha, H. S. NaNbO3/ZnO Piezocatalyst for Non-Destructive Tooth Cleaning and Antibacterial Activity. iScience 2022, 25 (9), 104915. https://doi.org/10.1016/j.isci.2022.104915.
[3] Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.; Rodríguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10 (12), 1828. https://doi.org/10.3390/w10121828.
[4] Ishibashi, K.; Nosaka, Y.; Hashimoto, K.; Fujishima, A. Time-Dependent Behavior of Active Oxygen Species Formed on Photoirradiated TiO2 Films in Air. J. Phys. Chem. B 1998, 102 (12), 2117–2120. https://doi.org/10.1021/jp973401i.
[5] Khan, Y.; Khan, M. N.; Salam, A.; Sadia, H.; Ullah, M. F.; Khan, M. I.; Abdullaeva, B. S.; Awwad, F. A.; Ismail, E. A. A. Photocatalytic Treatment of Organic Dyes Using Metal Oxides and Nanocomposites: A Quantitative Study. Open Chem. 2024, 22 (1), 20240026. https://doi.org/10.1515/chem-2024-0026.
[6] Zhang, L.; Mohamed, H. H.; Dillert, R.; Bahnemann, D. Kinetics and Mechanisms of Charge Transfer Processes in Photocatalytic Systems: A Review. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13 (4), 263–276. https://doi.org/10.1016/j.jphotochemrev.2012.07.002.
[7] Hernández-Alonso, M. D.; Fresno, F.; Suárez, S.; Coronado, J. M. Development of Alternative Photocatalysts to TiO2: Challenges and Opportunities. Energy Environ. Sci. 2009, 2 (12), 1231. https://doi.org/10.1039/b907933e.
[8] Akakuru, O. U.; Iqbal, Z. M.; Wu, A. TiO2 Nanoparticles: Properties and Applications.
[9] Nkwachukwu, O. V.; Arotiba, O. A. Perovskite Oxide–Based Materials for Photocatalytic and Photoelectrocatalytic Treatment of Water. Front. Chem. 2021, 9, 634630. https://doi.org/10.3389/fchem.2021.634630.
[10] Jiang, T.; Wang, Y.; Cai, C.; Nie, C.; Peng, H.; Ao, Z. Piezocatalysis for Water Treatment: Mechanisms, Recent Advances, and Future Prospects. Environ. Sci. Ecotechnology 2025, 23, 100495. https://doi.org/10.1016/j.ese.2024.100495.
[11] Qurbani, K.; Amiri, O.; Hamzah, H. Piezocatalysts as Antimicrobial Agents: A Promising Frontier in Pathogenic Bacteria Control. J. Hazard. Mater. Adv. 2025, 17, 100546. https://doi.org/10.1016/j.hazadv.2024.100546.
